
About
Dr. Komatsu is a senior scientist in the Johns Hopkins All Children's Department of Surgery and the Cancer & Blood Disorders Institute. He also has a secondary affiliation with the Johns Hopkins All Children’s Institute for Fundamental Biomedical Research. He is an associate professor in the Johns Hopkins University School of Medicine Department of Orthopaedic Surgery.
He earned an undergraduate degree in marine science/biology and a Ph.D. in cell biology at the University of Miami, where he also did post-doctoral training in immunology. He continued post-doctoral studies under Erkki Ruoslahti, M.D., Ph.D., at the Sanford Burnham Prebys Medical Discovery Institute.
Dr. Komatsu became an assistant professor in the University of Alabama at Birmingham Department of Pathology while maintaining an adjunct position with Sanford Burnham. He joined Sanford Burnham full time in 2008 before coming to Johns Hopkins All Children’s in 2018. He holds patents related to the R-RAS protein and peptide-mediated vascular targeting of pulmonary arterial hypertension (PAH).

Post-doctoral opportunity
A postdoctoral position is available in the laboratory of Masanobu Komatsu, Ph.D. at Johns Hopkins School of Medicine/All Children’s Hospital to study the tumor immune environment. High endothelial venules (HEV) are blood vessels specialized for recruiting naïve T cells and B cells. They serve a role as the gateways for lymphocyte entry into tumors and facilitate anti-tumor immune response.
Candidates with a relevant training background in immunology or vascular biology should apply. Interested applicants should submit a single PDF file containing CV and a brief description of research interests and accomplishments to Dr. Masanobu Komatsu at [email protected]
Education
- B.S., Biology and Marine Science, Minor: Mathematics, University of Miami, Miami, 1991
- Ph.D., Molecular Cell and Developmental Biology, University of Miami, School of Medicine, Miami. Mentor: Kermit L. Carraway, Professor. Thesis topic: Multifunctional activities and contributions of sialomucin complex/MUC4 to tumor metastasis. 1998
- Postdoc Associate, Dept. of Cell Biology and Anatomy, School of Medicine, University of Miami, Miami. Mentor: Kermit L. Carraway, Professor. Research topic: Multifunctional activities and contributions of sialomucin complex/MUC4 to tumor metastasis. 1998
- Postdoc Associate, Dept. of Microbiology and Immunology, School of Medicine, University of Miami, Miami. Mentor: Robert B. Levy, Professor. Research topic: Cytotoxic pathways of the host resistance to hematopoietic stem cell allograft. 1999 to 2000
- Postdoc Associate, The Burnham Institute, La Jolla, California. Mentor: Erkki Ruoslahti, Distinguished Professor. Research topic: Physiological and pathological roles of small GTPase R-Ras in vascular remodeling and regeneration. 2001 to 2004
Department and Institute Affiliations
- Cancer & Blood Disorders Institute, Johns Hopkins All Children’s Hospital
- Department of Surgery, Johns Hopkins All Children’s Hospital
- Department of Orthopaedic Surgery, Johns Hopkins University School of Medicine
- Institute for Fundamental Biomedical Research, Johns Hopkins All Children’s Hospital
Honors and Awards
- Article selected by the Faculty of 1000. Peptide-Directed Highly Selective Targeting of Pulmonary Arterial Hypertension. Am J Pathol., 2011
- Article selected by the Faculty of 1000. R-Ras is a global regulator of vascular regeneration that suppresses intimal hyperplasia and tumor angiogenesis. Nat Med, 2005
- Ruth L. Kirschstein National Research Service Award, NIH 5 T32 CA 09579, 2001-2002
- Winner, Sylvester Cancer Center Research Competition, 2000
- Third place, Sylvester Cancer Center Research Competition, 1999
- Winner, Sylvester Cancer Center Research Competition, University of Miami, 1998
- Top 10 most popular science news release of 2017
- Altmetric Score 98th percentile of all the tracked articles of a similar age in all journals and featured in 21 public news outlets. R-Ras-Akt axis induces endothelial lumenogenesis and regulates the patency of regenerating vasculature. Nature Communications, 2017
- Article selected by the Faculty of 1000. Peptide-Directed Highly Selective Targeting of Pulmonary Arterial Hypertension. American Journal of Pathology, 2011
- Article selected by the Faculty of 1000. R-Ras is a global regulator of vascular regeneration that suppresses intimal hyperplasia and tumor angiogenesis. Nature Medicine, 2005
- 2001-2002 Ruth L. Kirschstein National Research Service Award, National Institute of Health
Memberships
- American Association for Cancer Research
- American Association of Immunologists
- American Heart Association
- North American Vascular Biology Organization
- American Thoracic Society
Patents
- R-Ras activity in vascular regulation, U.S. Patent No. US8506965 B2
- Compositions containing a pharmacophore with selectivity to diseased tissue and methods of making same, U.S. Patent No. US20190022170A1
- CAR Peptide for Homing, Diagnosis & Targeted Therapy for Pulmonary and Fibrotic Disorders, U.S. Patent No. US9180161 B2
Research Interests
The overall mission of Dr. Komatsu’s laboratory is to take a multidisciplinary approach to address important unsolved problems in cancer, vascular disorders, and other diseases. Their broad research background including cancer immunology and vascular biology allows them to bring multiple disciplines together to basic research, clinical application, and technology development.
Dr. Komatsu's research interests include:
- Immuno-Oncology
- Drug Targeting
- Vascular Biology
Immuno-Oncology Program
Many malignant tumors are immune-suppressive or “immune cold”, which makes them unresponsive to cancer treatments.
The goal of this research is to convert immune cold tumors “immune hot” or immune responsive so that patient’s defense mechanism will destroy cancer cells and enhance efficacy of conventional chemotherapy and immunotherapy. Dr. Komatsu’s team explores novel strategies to do this by activating key innate and adaptive immune pathways.
Their current focus is ectopic lymphoid structures called tertiary lymphoid structures (TLS). The abundant TLS formation in patients’ tumors is strongly associated with therapy response and cancer survival. Dr. Komatsu’s research team has discovered that simultaneous activation of STING and lymphotoxin-β receptor pathways induces fully functional TLS in TLS-free tumors and provides protection against future recurrence and metastasis, enabling complete cure. Using this approach, functional TLS can be induced without any preconditioning, in different tumor types and anatomical sites, not restricted to certain organs such as the immune environment of the lung.
The team demonstrated that “spicing up” the tumor immune environment with STING activation improves the “fitness” of TLS and dramatically enhances B cell and T cell responses against tumors. Dr. Komatsu’s team is further investigating the mechanism of action of TLS-based therapy and developing its clinical use in adult and pediatric cancer treatment.
Drug Targeting Program
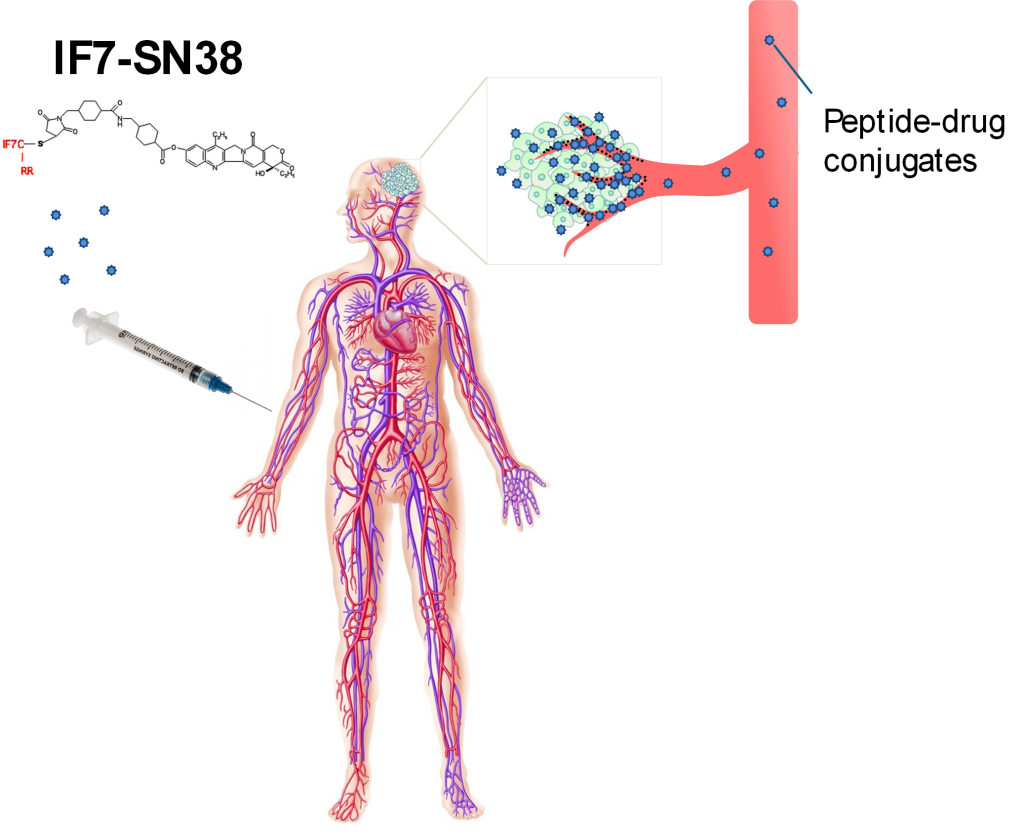
The other research program of Dr. Komatsu's laboratory is to develop and examine novel vascular targeting strategies for target-specific drug delivery guided by specific peptides. Peptide-directed vascular targeting takes advantage of unique molecular signatures of blood vessels at specific locations in the body.
Originally developed by Dr. Erkki Ruoslahti, this technology enables the direct delivery of drugs to tumors or other diseased tissues through the vascular network.
Since drugs are targeted to specific disease sites, it is possible to enhance the drug efficacy while substantially reducing unwanted adverse side effects. In collaboration with IF7Cure, Dr. Komatsu’s team is testing IF7-SN38, an IF7 peptide-conjugated chemotherapeutic, in a veterinary clinical trial of pet dogs with cancer. This trial is conducted at the Veterinary Clinical Trials Network of Johns Hopkins University led by the medical director, Dr. Rebecca Krimins, D.V.M. Previously, Dr. Komatsu’s team used another peptide called CAR peptide to successfully target lung lesions of pulmonary arterial hypertension (PAH). This peptide has been shown to be also useful to deliver anti-inflammatory medicines to the inflamed blood vessels thereby preventing severe organ damages in sepsis.
Vascular Biology Program
Malfunction and malformation of blood vessels are associated with a broad range of medical conditions, including cancer, cardiovascular diseases, and neurological disorders.
The goal of this research is to find a way to reverse the process of abnormal vessel formation and restore normal function to these vessels.
Normalization of blood vessels provides unique therapeutic opportunities. It can enhance the efficacy of cancer treatments and potentiate anti-tumor immunity, reestablish blood flow to ischemic hearts and limbs, and prevent blindness caused by diabetic retinopathy or macular degeneration.
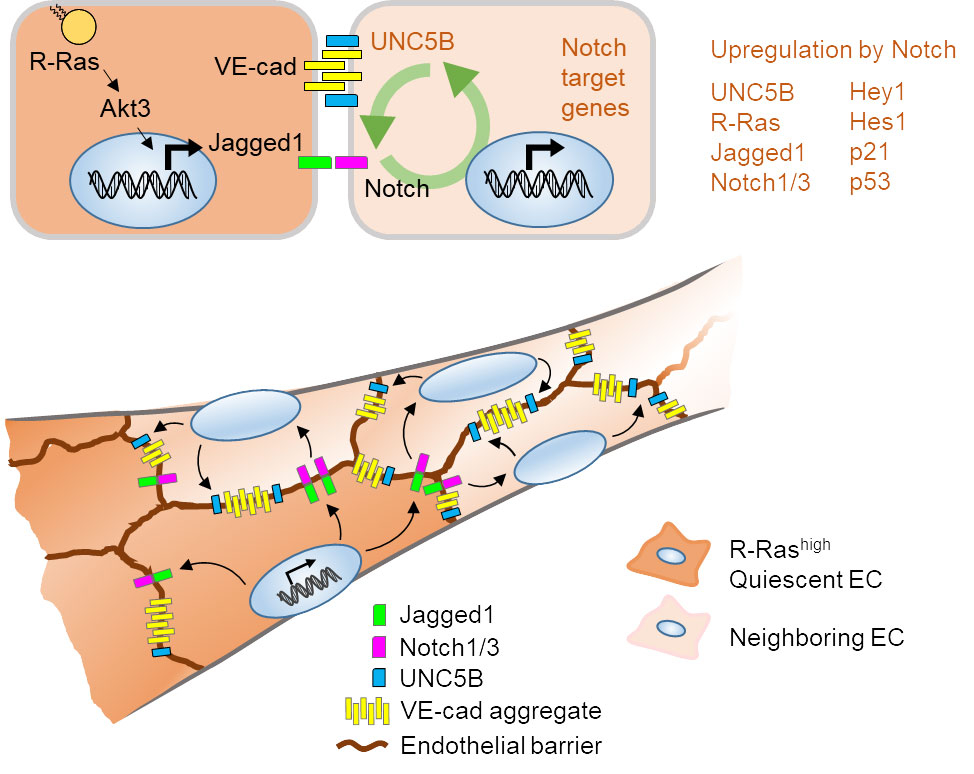
Dr. Komatsu's research team has identified a key role for the small GTPase R-Ras in promoting vessel maturation while preventing excessive angiogenic responses in regenerating vasculature. Unlike K-Ras or H-Ras, R-Ras inhibits vascular cell proliferation and invasion and promotes quiescence. The elevated R-Ras expression normalizes pathologically regenerating blood vessels. There is currently no successful therapeutic strategy for vascular normalization.
Dr. Komatsu's team showed that R-Ras coordinates multiple signaling events in endothelial cells and pericytes to redirect nascent vessel formation from angiogenic sprouting to vessel stabilization. The unique activities of R-Ras make this Ras homolog an important subject of investigation in search of a new strategy for manipulating blood vessel function.
Research Focuses:
- Immuno-Oncology
- Drug Targeting
- Vascular Biology
Research Aims:





Selected Publications
- Akt3 activation by R-Ras in an endothelial cell enforces quiescence and barrier stability of neighboring endothelial cells via Jagged1. Herrera JL and Komatsu M. Cell Reports. 2024; 43(3):113837. PMID: 38402584
- Molecular signature of tumor-associated high endothelial venules that can predict breast cancer survival. Sawada J, Hiraoka N, Qi R, Jiang L, Fournier-Goss AE, Yoshida M, Kawashima H, Komatsu M. Cancer Immunology Research. 2022; 10(4):468-481. PMID: 35201289
- Partial RAG deficiency in humans induces dysregulated peripheral lymphocyte development and humoral tolerance defect with accumulation of T-bet+ B cells. Csomos K, Ujhazi B, Blazso P, Herrera JL, Tipton CM…Svaton M, Ward BR, Komatsu M, Kumanovics A, Butte MJ...Walter JE. Nature Immunology. 2022; 23(8):1256-1272. PMID: 35902638
- Prostaglandin E2 breaks down pericyte-endothelial cell interaction via EP1 and EP4-dependent downregulation of pericyte N-cadherin, connexin-43, and R-Ras. Perrot C, Herrera J, Fournier-Goss A, and Komatsu M. Scientific Reports. 2020; 10(1):11186. PMID: 32636414
- R-Ras-Akt axis induces endothelial lumenogenesis and regulates the patency of regenerating vasculature. Li F, Sawada J, and Komatsu M. Nature Communications. 2017; 8(1):1720. PMID: 29170374
- Small GT Pase R-Ras regulates integrity and functionality of tumor blood vessels. Sawada J, Urakami T, Li F, Urakami A, Zhu W, Fukuda M, Li DY, Ruoslahti E, Komatsu M. Cancer Cell. 2012 Aug 14;22(2):235-49. PMID: 22897853

