About
Dr. Nagy is a professor of medicine, biological chemistry and biomedical engineering in the Division of Endocrinology, Diabetes and Metabolism in the Department of Medicine in the Johns Hopkins University School of Medicine. He is the associate director of the Johns Hopkins Center for Metabolic Origins of Disease, a program that spans Johns Hopkins Medicine campuses in St. Petersburg, Florida, and Baltimore.
He is also co-director of the Johns Hopkins All Children's Institute for Fundamental Biomedical Research. He has training as both a physician and as a molecular and cellular biologist.
Dr. Nagy’s research, in broad terms, focuses on dissecting and understanding how the identity of cells develops and how their specialization contributes to human diseases. He seeks to learn how the extra- and intracellular lipid environment contributes to cellular development and differentiation, and what impact that has on components of the immune system. In this context, Dr. Nagy also studies what makes cells to use certain pieces of their genetic information and not others, and what causes that process to sometimes result in diseases such as chronic inflammation, tissue degeneration or cancer. Studying these questions while evaluating the entire genome makes it more likely to discover key changes related to a particular disease and to find reliable biomarkers to monitor disease progression. The answers he obtains may lead to better diagnoses and novel therapies.
Dr Nagy published around 200 research and review articles for which he received circa 28,000 citations and has an h-index of 66. His research has been consistently funded by the NIH.
Education
- M.D., University Medical School of Debrecen, Debrecen, Hungary, 1991
- Ph.D., University Medical School of Debrecen, Debrecen, Hungary, 1995
Department and Institute Affiliations
- Institute for Fundamental Biomedical Research, Johns Hopkins All Children’s Hospital
- Johns Hopkins Center for Metabolic Origins of Disease
- Department of Medicine, Division of Endocrinology, Diabetes and Metabolism, Johns Hopkins University School of Medicine
- Department of Biological Chemistry, Johns Hopkins University School of Medicine
Honors and Awards
Dr. Nagy is the recipient of numerous awards, including a Boehringer Ingelheim Research Award, a Wellcome Trust Senior Research Fellowship in Biomedical Sciences, and three consecutive Howard Hughes Medical Institute International Research Scholar Awards.
He is an elected member of the Hungarian Academy of Sciences, the European Molecular Biology Organization (EMBO), Academia Europaea and The Henry Kunkel Society.
Research Interests
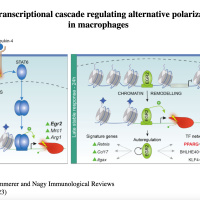
Transcriptional and epigenomic control of macrophage polarization:
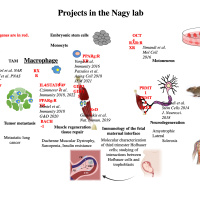
We are interested in understanding how macrophages reach and maintain their cellular phenotype and effector functions. To this end we study the mechanisms of interplay between transcription factor binding, enhancer activity and the 3D genome conformation with a focus on nuclear receptor and heme signaling. Our laboratory utilizes diverse experimental techniques such as RNA-Seq, ChIP-Seq, ATAC-Seq, Hi-ChIP. HiC, lipidomics, bioinformatics and data integration and in vivo injury and disease models in skeletal muscle as well as lung regeneration.
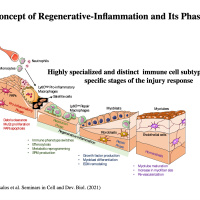
Regenerative inflammation:
We are also interested in identifying the role of inflammation and inflammatory cells primarily macrophages in the physiological function, disease progression and as therapeutic targets in skeletal muscle pathology (i.e. Duchenne Muscular Dystrophy, sarcopenia), the lung (Acute Lung Injury) and the human placenta (i.e. preeclampsia). Our research projects involve single cell and spatial transcriptomics and subtype specification of myeloid cells during tissue repair, regenerative inflammation in acute and chronic inflammation in skeletal muscle, the lung and the human placenta. Much of this work has clinical and translational relevance and we are exploring ways to apply biomedical engineering principles and approaches to alter disease progression and pave the way towards novel therapeutics.
Research Focuses:
Research Aims:
Selected Publications
- Andreas Patsalos, Laszlo Halasz, Miguel A. Medina-Serpas, Wilhelm K. Berger, Bence Daniel, Petros Tzerpos, Mate Kiss, Gergely Nagy, Cornelius Fischer, Zoltan Simandi, Tamas Varga, Laszlo Nagy. A growth factor–expressing macrophage subpopulation orchestrates regenerative inflammation via GDF-15. J Exp Med (2022) 219 (1): e20210420. doi: 10.1084/jem.20210420 - VIDEO ABSTRACT
- Bence Daniel, Zsolt Czimmerer, Laszlo Halasz, Pal Boto, Zsuzsanna Kolostyak, Szilard Poliska, Wilhelm K. Berger, Petros Tzerpos, Gergely Nagy, Attila Horvath, György Hajas, Timea Cseh, Aniko Nagy, Sascha Sauer, Jean Francois-Deleuze, Istvan Szatmari, Attila Bacsi, and Laszlo Nagy. The transcription factor EGR2 is the molecular linchpin connecting STAT6 activation to the late, stable epigenomic program of alternative macrophage polarization. Genes Dev. 2020 Oct 15;34(21-22):1474–92. doi: 10.1101/gad.343038.120. - VIDEO ABSTRACT
- Patsalos A, Tzerpos P, Halasz L, Nagy G, Pap A, Giannakis N, Lyroni K, Koliaraki V, Pintye E, Dezso B, Kollias G, Spilianakis CG, Nagy L. The BACH1-HMOX1 Regulatory Axis Is Indispensable for Proper Macrophage Subtype Specification and Skeletal Muscle Regeneration. J Immunol. 2019 Sep 15;203(6):1532-1547.—VIDEO ABSTRACT
- Giannakis N, Sansbury BE, Patsalos A, Hays TT, Riley CO, Han X, Spite M, Nagy L. Dynamic changes to lipid mediators support transitions among macrophage subtypes during muscle regeneration. Nat Immunol. 2019 Apr 1. (Epub ahead of print)—VIDEO ABSTRACT
- Daniel B, Nagy G, Czimmerer Z, Horvath A, Hammers DW, Cuaranta-Monroy I, Poliska S, Tzerpos P, Kolostyak Z, Hays TT, Patsalos A, Houtman R, Sauer S, Francois-Deleuze J, Rastinejad F, Balint BL, Sweeney HL, Nagy L. The nuclear receptor PPARγ controls progressive macrophage polarization as a ligand-insensitive epigenomic ratchet of transcriptional memory. Immunity. 2018 Oct 16;49(4):615-626. PMCID: PMC6197058—VIDEO ABSTRACT
- Czimmerer Z, Daniel B, Horvath A, Rückerl D, Nagy G, Kiss M, Peloquin M, Budai MM, Cuaranta-Monroy I, Simandi Z, Steiner L, Nagy B Jr, Poliska S, Banko C, Bacso Z, Schulman IG, Sauer S, Deleuze JF, Allen JE, Benko S, Nagy L. The Transcription Factor STAT6 Mediates Direct Repression of Inflammatory Enhancers and Limits Activation of Alternatively Polarized Macrophages. Immunity. 2018 Jan 16;48(1):75-90.e6. PMCID: PMC5772169—VIDEO ABSTRACT
- Varga T, Mounier R, Patsalos A, Gogolák P, Peloquin M, Horvath A, Pap A, Daniel B, Nagy G, Pintye E, Póliska S, Cuvellier S, Larbi SB, Sansbury BE, Spite M, Brown CW, Chazaud B, Nagy L. Macrophage PPARγ, a lipid activated transcription factor controls the growth factor GDF3 and skeletal muscle regeneration. Immunity. 2016 Nov 15;45(5):1038-1051. PMCID: PMC5142832